-
 No. 78 Zhancheng Avenue, Taozhu Street, Zhuji, Zhejiang,CN
No. 78 Zhancheng Avenue, Taozhu Street, Zhuji, Zhejiang,CN
-
 +86-0575-87077868
+86-0575-87077868








Instructions for Surgical Masks
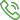
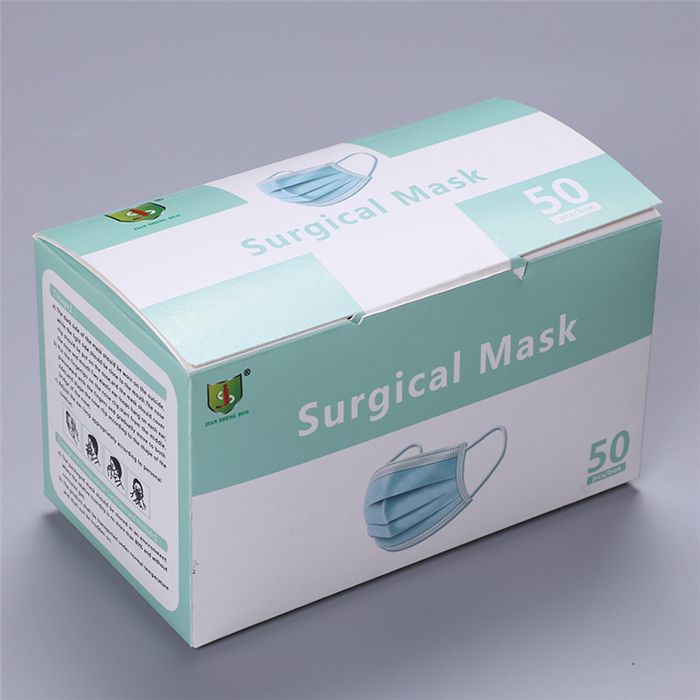
[Product Name]:Surgical mask
[Product Model/Specification]:Sterile,ear-loop、Non-sterile,ear-loop;17.5cm*9.5cm
[Scope of Application]:It covers the user's mouth, nose and jaw to provide a physical barrier against the direct penetration of pathogens, microorganisms, body fluids, particles, etc.
[Raw Materials,Structure and Composition of the Product]:
The product consists of a mask body, a nose clip and a mask band.The mask body is divided into three layers: inner and outer layers are polypropylene (PP) non-woven cloth, and the middle layer is polypropylene (PP) melt-sprayed cloth.The nose clip is made of galvanized iron wire with PE material.The mask band is spandex.
[Product Performance/Implementation Standards]:YY 0469-2011 Surgical Mask(Non-sterile,ear-loop type, except 4.9.2 sterility (not applicable), 4.10 residual ethylene oxide (not applicable), 4.11 skin irritation, 4.12 cytotoxicity and 4.13 delayed type hypersensitivity;Sterile,ear-loop type,excpt 4.9.1 microbe, 4.11 skin irritation, 4.12 cytotoxicity and 4.13 delayed type hypersensitivity).
[Product Contraindication]:None.
[Usage]:
How to wear mask:
a) The dark side of the mask should be worn on the outside,while the light side should be close to the mouth.The nose clip should be put on the nose,and the mask should cover the nose and mouth.Two elastic ear-loops hang on each ear.
b) Put the fingertips on the nose clip,start from the middle,pressnward with the fingers and gradually move to both sides,and shape the nose clip according to the shape of the bridge of the nose;
c) Adjust the ear-loop appropriately according to personal comfort.
How to remove mask:
a) Do not touch the front of the mask (contaminated surface) .
b) Remove elastic bands from both ears.
c) The elastic band used to hold the mask only is thrown into the clinical waste container.
[Precautions]:
a) The product is disposable,and it is prohibited to reuse;
b) Check the integrity of the package before using.If any damage is found,it is strictly prohibited to use;
c) Sterile products are sterilized with ethylene oxide;
d) Use the product as soon as possible after opening;
e) The mask should be replaced in time after it becomes damp and contaminated by the patient’s blood and body fluids;
f) The products shall be disposed of in accordance with the requirements of the Regulations on The Management of Medical Waste after using.
g) Those that have not been sterilized should not be used in environments where sterility is required.
h) The product is flame retardance.
[Sample Diagram of Label and Package Identification]:
indicates that the product can only be used once.
[Storage and Transportation Conditions and Methods]:
a) The packaged mask should be stored in an environment where the relative humidity is no more than 80% and without corrosive gas;
b) The product shall be transported under normal temperature and shall not be trodden on.
[Production Batch Number]:See the packaging label
[Production Date]:See the packaging label
[Term of use]:12 months from the date of production
[Registrant name]:Zhejiang Jiansheng Medical Products Co.,LTD
[Domicile of registrant]:Building 23,No.78,Zhancheng Avenue,Taozhu Street,Zhuji City,Zhejiang Province
[Contact Number]:(0086)18888757333
[Name of manufacturer]:Zhejiang Jiansheng Medical Products Co.,LTD
[Residence of manufacturer]:Building 23,No.78,Zhancheng Avenue,Taozhu Street,Zhuji City,Zhejiang Province
[Production Address]:Building 22,No.78,Zhancheng Avenue,Taozhu Street,Zhuji City,Zhejiang Province
[Contact Number]:(0086)18888757333
[Postal Code]:311800
[After-sales Service unit]:Zhejiang Jiansheng Medical Products Co.,LTD
[Production Enterprise License No.]:ZSYJX Production License No.20200129
[Medical Device Registration Certificate No.]: ZXZZ20202141440
[Product Technical Requirement Number]:/
[Date of Preparation of specification]:February 1,2020
[Others]:For epidemic prevention and control emergency use only